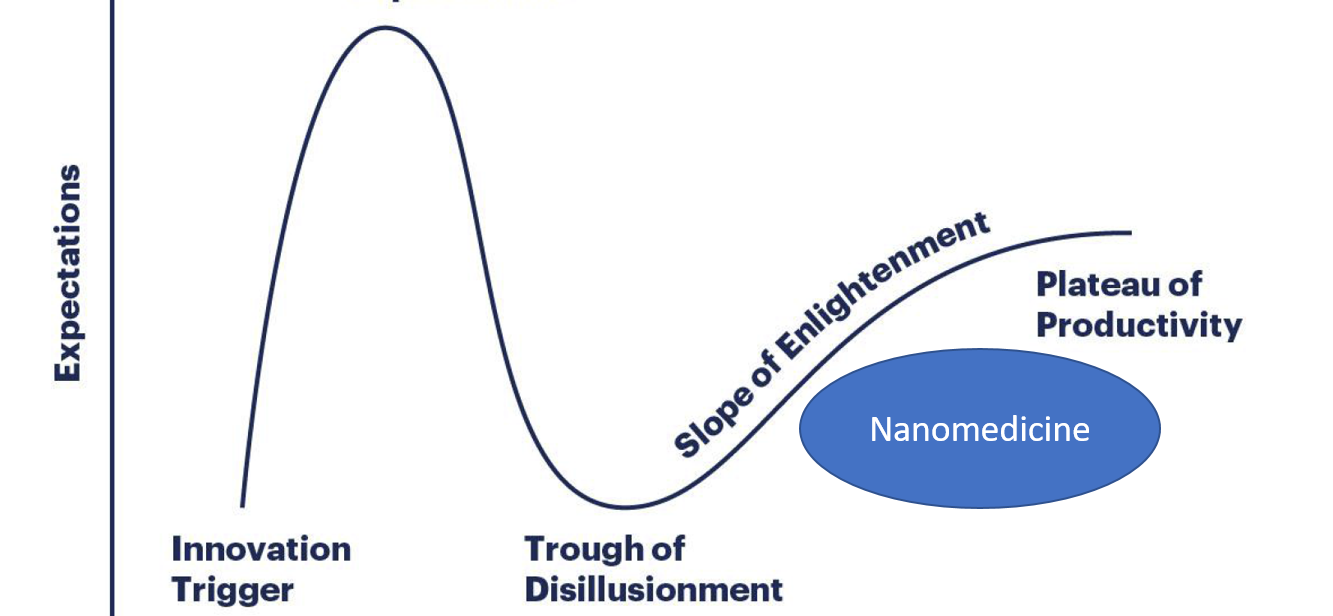
Where is the field of nanomedicine today?
During the last months several headlines and commentaries have aimed to address the status of the field of nanomedicine. Specifically, it has been questioned whether we were “at the beginning of the end” of what is provocatively presented as the hype of this novel technology. The so-called Gartner Hype Cycle has been used as a model of evolution and adaptation of novel technologies. The figure shows its various states a new technology passes from innovation trigger to the eventual plateau of productivity.
Peak expectations:
Following the clinical introduction of Doxil® in 1995, and its success in the market, the field of nanomedicine ballooned and diversified into development of a range of novel materials and intricate formulations. The promise of active nanoparticle targeting to disease sites proved particularly challenging to fulfil and, combined with a heavy rely on the enhanced permeation retention effect for disease site accumulation of nanoparticles, lead some to express doubts on the value of nanomedicines in general.
Important and decisive FDA approvals
10 days ago, Alnylam Pharmaceuticals announced the FDA approval of GIVLAARI™ (givosiran), an RNA interference (RNAi) therapeutic for the treatment of adults with acute hepatic porphyria, an ultra-rare, genetic disease (http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-approval-givlaaritm-givosiran-us-food-and-drug). This is the second RNAi therapeutic reaching the clinic in a time-period of only 16 months. In August 2018, FDA approved the first-ever RNAi therapeutic, ONPATTRO™ (patisiran) for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults, another rare genetic disease (http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-first-ever-fda-approval-rnai-therapeutic). Not only are these products the first-in-class RNAi drugs reaching the market. They are a landmark event for the advancement of precision genetic medicines, opening for novel treatment options for rare diseases with limited cures available and their translation was only possible through a nano-based formulation, solid lipid nanoparticles. This demonstrates the crucial and ground-breaking contributions of nanotechnology to novel treatments, enabling the clinical translation of a whole new class of medicines, i.e. RNAi therapeutics. There is substantial hope and expectations that nanomedicine will also contribute to the translation of other novel medicines, e.g. mRNA therapeutics.
Optimism
We are not at the “beginning of the end” of the nanomedicine era. Nanomedicine is getting established in the “slope of enlightenment” of the Gartner Hype Cycle for New Technologies on the right track towards the “Plateau of Productivity”. Nanomedicine is at a turn-around from academic discovery to industrial development and clinical implementation. We see a change towards nanomedicine being an established tool in the toolbox to address health challenges, rather than a ‘fancy’ scientific novelty.
Hurdles and gaps still to fill
Still there are some main hurdles in clinical translation of nanomedicines: examples are (i) the lack of batch-to-batch reproducibility, (ii) the complexity of the manufacturing processes, (iii) the lack of appropriate characterization protocols and (iv) poorly defined critical quality attributes, as well as the absence of (v) clinically relevant animal models. The Open Innovation Test Bed EU-project SAFE-N-MEDTECH aims to build pipelines and support structures to help European industry meet these challenges and bring new nanotechnology-based therapeutics to the market and to the patients