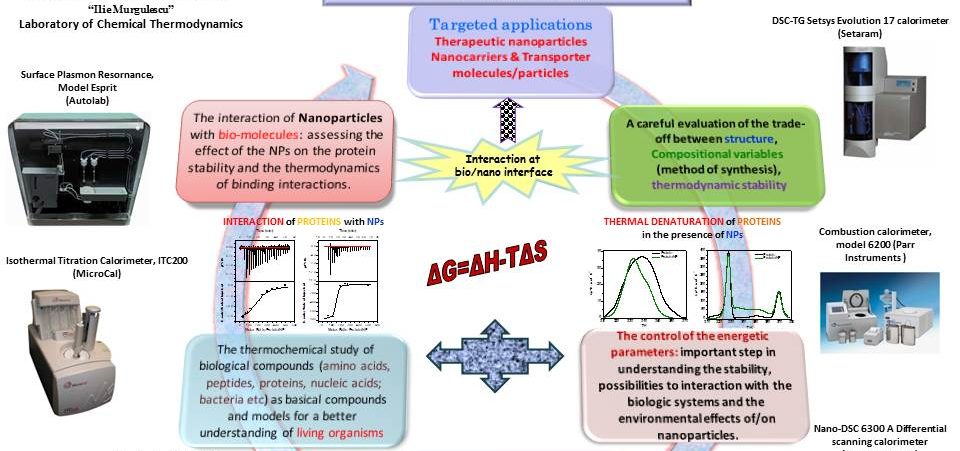
The understanding of molecular interaction and the identification of ligands which have an appropriate biological effect on a validated target biomolecule is a central point in the research activity with impact in the advanced or nano-enabled drug delivery systems. In order to elicit a biological effect, a ligand must bind to the target biomolecule with an appropriate affinity and have suitable physicochemical properties. Such analyses need the thermodynamic data, because the driving forces for chemical reactions and diffusion can be given properly in terms of thermodynamic properties. That is why the thermodynamic characterization of binding interactions at various stages in the rational drug design process is essential for advances in this field. Chemical Thermodynamics with all applied thermoanalytical methods could be a valuable tool in understanding the complex phenomena occurring at the bio/nano interface.
The choice of the therapeutic entity could include: proteins, antibodies, nucleic acids, antigens, peptides, PNAs, foldamers etc. The principal targeted applications: ∙ Therapeutic nanoparticles; ∙ Nanocarriers & Transporter molecules/particles.
Major objectives:
• Thermodynamic characterization of binding interaction at bio/nano interface
Understanding the thermodynamic contributions during the interaction at bio/nano interface is the key to driving rational drug discovery. By far the most reliable route to this characterization is the direct measurement of the binding enthalpy, which may be accomplished by means isothermal titration calorimetry (ITC), a modern method which can measure interaction heats with great accuracy. Other energetic parameters and thermodynamic contributions could be also evaluated: binding constant; binding stoichiometry; the entropy and Gibbs free energy changes arising from the binding interaction. The changes of enthalpy and entropy of binding are being considered as the driving factors for binding, their contributions to the free energy of binding being closely related, according to Gibbs law of free energy: ΔG=ΔH-TΔS, where ΔG, ΔH and ΔS are the changes of Gibbs free energy, enthalpy and entropy upon binding, and T is the absolute temperature. Understanding the thermodynamic contributions to free energy changes, and therefore affinity measurements, is key to driving rational drug discovery. Also within the scope of measuring of protein–ligand binding kinetics and affinities, Surface Plasmon Resonance (SPR) is used. This technique is an optical-based method to measure the change in the refractive index near a sensor surface, is label-free and capable of measuring the real-time binding data.
• The investigation of the protein stability (folding/unfolding) in the presence of nanoparticles, as well as of the ligand induced conformational changes
Designing of a drug or elucidate the mechanism of biochemical interactions requires the understanding the manner in which chemical and structural characteristics of the nanosystems affect the binding specificity and complex integrity ligand-macromolecule. Ligand induced conformational changes can have a key role in mechanism of biomolecular binding interaction being responsible for correct orientation of active site, or the subsequent binding of ligands such as further substrates or allosteric effectors. The unfolding process and the conformational changes are subtle processes so that their investigation will be performed by using ultrasensitive differential scanning calorimeter (Nano-DSC calorimeter). The thermodynamic results could be correlated with the results obtained by ITC, SPR, Circular Dychroism and UV-visible analysis, Conductivity measurements and pH measurements, as well as Fourier transformed infrared spectroscopy (FTIR).
• Key parameters to explain the chemical reactivity of nanocrystalline materials
Understanding the properties of nanoparticles presents a host of challenging questions and problems. A focus will be given to the energetic parameters and the understanding of their crossover when working at the nanolevel, as important steps in investigating the stability, possibilities to interaction with the biologic systems and the environmental effects of/on nanoparticles. In the project some key parameters involved in the control of the reactivity of nanoparticle will be analyzed: the relative contribution of particle size versus particle composition; the relative contribution of particle size versus crystalline structure; correlation between the stability, energetic parameters and the therapeutic effect of nanoparticles. For a complex and systematic study will be used a plethora of experimental methods (see: www.icf.ro). A careful evaluation of the trade-off between structure, compositional variables and thermodynamic data will have both applicative and predictive values enabling some decision making in ligand selection and/or optimization, the potential safety concerns being also addressed.
Edit

Menu
+34 622 000 000
angel.delpozo@keralty.com
Síguenos en
Copyright 2023 Safe-n-Medtech. All rights reserved.
Menu
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No 814607
Copyright 2022 Safe nmt. All rights reserved. Design Web Barcelona.