Biological nanoparticles with high potential for diagnostics and therapy
Extracellular vesicles (EVs) are nanosized, lipid-based membrane vesiclesreleased by cells constitutively or in a regulated manner. EVs carry a specific subset of proteins, nucleic acids, lipids and metabolites reflecting the type and condition of the cell they originate from. The release of EVs in all bodily fluids represents a biologically significant communication system. EVs can transfer their content to recipient cells, and this transfer of the molecular and genetic cargo is accompanied by the reprogramming of the recipient cell functions. For this reason, they have been foundattractive as mediators for therapeutic tissue regeneration and immune system modulation, in which case their molecular content can be engineered. As the EV-associated molecular cargoes found in biofluids contain a rich source of information about the cellular processes in both health and disease, they are also considered for early diagnostics and follow-up of a wide number of diseases, e.g. cancer, cardiovascular disorders and neurodegenerative diseases. By identification of disease-related EV subpopulations expressing specific biomarkers, EVs are promising to distinguish and stratify disease populations, and to enable the development of personalized treatment protocols.
Technological challenges
Considerable research efforts have been done to demonstrate the clinical value of EVs, but yet several barriers have to be crossed to enable the translation of extracellular vesicles as a clinical diagnostic or therapeutic tool. Besides optimal processing workflows for clinical samples to preserve relevant EV-associated information, improved technologies and methods are needed for isolation of pure, disease-related EV subsets, their characterization, quantification, and specific biomarker detection. These represent major challenges and opportunities for development and validation of innovative technologies. The main technical requisites for such systems are high-throughput, short turn-around time, automation, and ease of use. At a later stage and with respect to the In Vitro Diagnostics and Medical Devices Regulations, standardization and traceability of the methods and workflows, and compliance with safety and performance requirements are equally important.
Diagnostic target EVs are often outnumbered by EVs from healthy tissues, platelets or red blood cells, lipoproteins and other biomolecules abundantly present in liquid biopsies, such as plasma. Current methods for isolation of EVs from liquid biopsies include density gradient centrifugation, ultracentrifugation, affinity membranes, and size exclusion chromatography among others. These bulk methods are often time consuming and not efficient to obtain relevant EV subsets in sufficient quantities, importantly affecting the analysis outcome. Therefore, single-vesicle enrichment and characterization methods are preferable.
Nano-enabled technologies
Single-vesicle isolation and/or analysis hold great promise as they can directly provide information about rare sub-populations of EVs. However, the small size of EVs and low abundance of surface biomarkers challenges conventional clinical approaches, leading to the development of vesicle-specific instruments, assays and protocols. High-sensitivity flow cytometry is one such approach, potentially allowing the robust and quantitative sorting and simultaneous measurement of EV number, size, and per-particle surface marker expression with a wide dynamic range and high throughput.
For successful implementation of single-particle strategies, nanotechnologies have the potential to boost sensitivity and multi-parameter analysis. Properly designed and engineered nanobeads with immune-affinity binding properties not only allow for enrichment and sub-fractionation of EVs in complex liquid biopsy samples, but can also be applied as nanolabels with superior signal intensity (e.g. quantum dots). Furthermore, nanobeads with similar physico-chemical properties compared to extracellular vesicles (e.g. size, refraction index, surface hydrophobicity, surface-associated biomolecules) are being developed for calibration and standardization purposes.
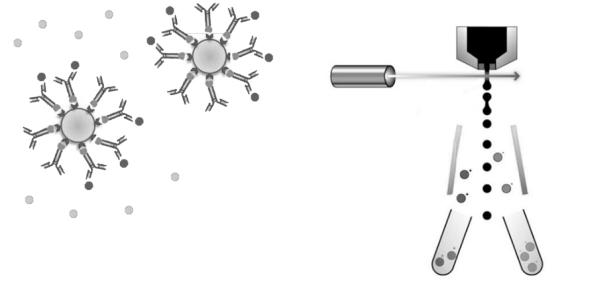
Figure: Nanobead-enabled enrichment (left) and high-sensitivity flow cytometry (right) strategies for sub-fractionation of EV-subsets
OITB Services
As one of the OITB Safe-N-Medtech partners VITO has expertise, state-of-the-art methods and fully equipped laboratories available for isolation and characterization of specific EV subsets from in vitro/ex vivo cell systems and clinical samples (plasma, urine, cerebrospinal fluid). Highlights of the technology platform are fluorescence-based nanoparticle tracking analysis (NanoSight NS500-HZGF, Particle Metrix ZetaView Twin) and small-particle flow cytometry (Becton Dickinson Influx and FACS Celesta SORP), as well as LC-MS-based proteomics analysis of clinical EV fractions.
VITO’s methods and equipment can be used for discovery of therapies and diagnostics, and development of production and quality processes.