Reutilization of single-use medical devices
Currently, a large part of the health centres uses surgical instruments labelled by the manufacturer as single-use material. This material, on the recommendation of the manufacturer, should be discarded through its initial use, becoming a biohazard waste. In this context, the need for financial savings of health centres and the ecological awareness that has increased in recent years, unleashed the reutilization of this material (In 2012, 45.2% of European hospitals with more than 250 beds, according to the FDA, performed this practice). This is defined as the use of a medical devices, more times than those specified by the manufacturer on the label and is normally preceded by reprocessing and remanufacturing, which includes all the steps taken to convert a contaminated single-use device into a device ready to be used in another patient. These stages are the following: washing, functionality tests, packaging, labelling and sterilization. Reprocessing is foreseen in the new EU legislation on medical devices, (Article 17).
Nanotechnology to improve surface properties
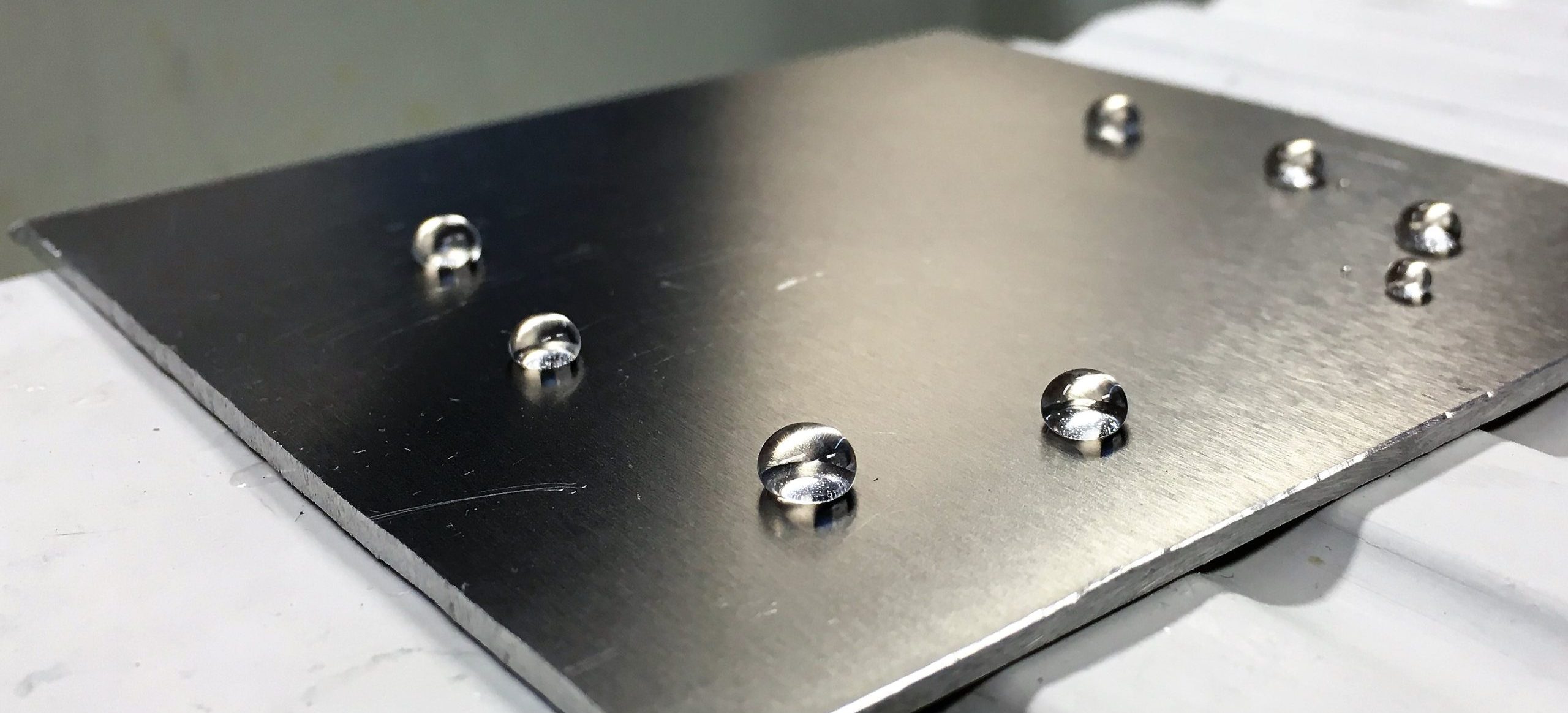
Practicality, innovation and high technology have allowed TECNAN to produce and commercialize singular products that have achieved to position themselves as leaders in their respective application areas. And as TECNAN is in constant evolution, listening to its customers and analysing trends, continuously discovering new applications and utilities of the developed products. One of these new applications that TECNAN is working on is the use of its ”ready to use” products as a coating for medical devices that allow an easier reutilization. Among the products developed and commercialized by TECNAN there is a range of products “ready to use” which creates a nanotechnological coat able to improve the properties of the surfaces where are applied. The coatings can be deposited in surfaces of different character (plastic, metallic or ceramic) conferring properties as anti-fouling effect, hydrophobicity and oleophobicity, scratch resistance and antimicrobial effect among others. In the case of the reutilization of single-use medical devices anti-fouling effect, can help facilitating the washing during the remanufacturing; antimicrobial effect can help reducing the risk of biological risk and scratch resistance can help to maintain the integrity and proper functioning of the device.
Participation in SAFEnmt
Nowadays, TECNAN is applying some of their nanocoatings to confirm the potential use of this technology to enhance reprocessing chances for the medical devices. Surgical instruments of different materials have been treated showing good results. It was found that easy-to-clean properties were achieved that would facilitate the cleaning processes in the reprocessing of the material and also allow including the antibacterial property of silica particles and hydroxyapatite in cooperation with CIBER. TECNAN has reference products in sectors such as automation and construction, however, the company has never applied its product for the medical sector and this is the reason why TECNAN is part of this amazing project. TECNAN is acting as test case for the OITB development, the services that can be of interest is the support for a well design of the product, market focus as well as advise and development in order carry out the process of successfully implementing the product in the healthcare market. For that, the company requires the advice of SAFENMT project on issues such as legislation, assessment of existing data, evaluation of the potential of nanoparticles leakage, biocompatibility, cytotoxicity, surface integrity and sterility, production and business model among others.
Interested in finding out more about TECNAN and its nanotechnological products? Visit the website: https://tecnan-nanomat.es/en/