Oncology Applications
Externally activated nanoparticles for cancer treatment
Resonant Circuits Limited (UK)
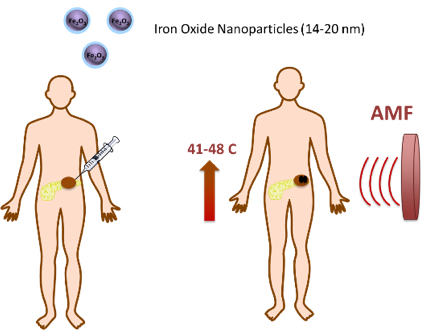
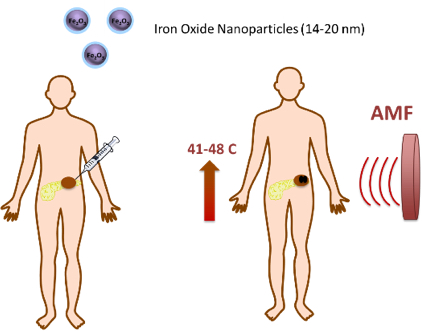
The starting point of this pilot case is the iron-oxide magnetic nanoparticles developed and validated in the framework of the H2020 NoCanther project. The iron oxide nanoparticle core can generate heat when subjected to an external alternating magnetic field (AMF), generated by the MACH® system, inactivating the tumor cells and leading to tumor shrinkage by physical means, being classified as a MD. This approach prevents resistance to chemotherapy, while creates a synergistic effect with anti-cancer drugs. The product is very versatile and can be adapted to any further conventional oncological treatments for localized tumors like sarcoma, liver, breast and colon cancers. Currently, it is under development for a clinical application in pancreatic cancer. The NoCanther product will benefit from these OITB services:
→ Regulatory advice for a combined product (nanoparticles+MACH® system)
→ HTA to compare against current treatments
→ Validation of the physic-chemical characterization already performed (this will work also to check the validity of the methods developed by the OITB)
→ Endotoxins determination
→ Preclinical safety and efficacy testing in other types of cancer (new indications)
→ Pilot production
→ Clinical evaluation
→ Business models for new indications (e.g. colorectal cancer)
→ Assistance with the MACH® system prototyping and regulatory approval
This case will mean a huge challenge for the OITB, as it is one of the more complicated MDs to be assessed and translated (injectable nanoparticles and external activation).